The Law of Conservation of Mass asserts that in any closed system, you’ll find the total mass remains constant regardless of the transformations substances undergo. This fundamental principle highlights that mass is neither created nor destroyed; it’s merely transformed. Credited to Antoine Lavoisier in the 18th century, this law serves as a cornerstone in scientific theories, particularly in chemical reactions and environmental science, ensuring predictable results. Your grasp of this concept will enhance as you explore its application in real-world scenarios, from ecological sustainability to precise chemical engineering. There’s always more to uncover with further exploration.
Key Takeaways
- The Law of Conservation of Mass states that mass cannot be created or destroyed in a closed system.
- During any chemical reaction, the total mass of the reactants equals the mass of the products.
- This law was first formulated by Antoine Lavoisier in the 18th century through meticulous experiments.
- It is fundamental in understanding chemical equations, ensuring that atoms are balanced on both sides of the reaction.
- In practical terms, this law implies that in any physical or chemical process, the amount of mass remains constant.
Defining Conservation of Mass
The law of conservation of mass states that in a closed system, the total mass remains constant, regardless of the processes occurring within the system. This principle hinges critically on the concept of mass equivalence, asserting that mass can neither be created nor destroyed; it merely transforms from one form to another.
To comprehend and verify this law, you’ll rely on advanced measurement techniques. Precision in these techniques is paramount, as even slight errors can lead to significant misinterpretations of the law. One common method involves the use of a closed container where a reaction takes place, ensuring no mass is lost to the surroundings. Here, sensitive scales measure the mass before and after the reaction, confirming if mass is conserved.
Moreover, understanding the subtleties of mass equivalence is crucial. It implies that all mass within a system, when measured precisely, will account for the same total at any point in time, provided no mass has entered or left the system.
This concept is foundational in fields such as chemistry and physics, where it supports fundamental laws, including the conservation of energy, given the interrelationship between mass and energy in the physical world.
Historical Development
As you explore the historical development of the Law of Conservation of Mass, you’ll first encounter early scientific theories that preceded modern understanding.
Antoine Lavoisier’s groundbreaking experiments in the late 18th century, which meticulously quantified the mass of reactants and products, were pivotal. These experiments provided the empirical evidence needed to definitively support the law, challenging and refining previously held notions.
Early Scientific Theories
Understanding early scientific theories reveals how initial misconceptions about mass and matter evolved into the foundational principles we recognize today. You must consider the profound impact of Greek philosophy on these early ideas.
The Greeks, including philosophers like Democritus and Aristotle, posited theories about the nature of matter that significantly diverged from modern scientific understanding. Democritus introduced the concept of indivisible units, or atoms, which laid groundwork, yet Aristotle’s belief in four fundamental elements—earth, water, air, and fire—predominated and influenced scientific thought for centuries. These elements, he argued, could transform into each other, a belief that inherently contradicted the later established law of conservation of mass.
Alchemical practices further complicated the early exploration of mass and matter. Alchemists, driven by the goals of transmutation and the elixir of life, engaged in processes that seemed to support the transformation of substances into entirely different forms. Their meticulous experiments, though often shrouded in mysticism, inadvertently set the stage for a more empirical approach to understanding matter. They observed changes in physical properties and mass during their transformations, adding layers of complexity to the study of matter before the concept of mass conservation was precisely defined.
Lavoisier’s Crucial Experiments
Building on the alchemical backdrop, Antoine Lavoisier conducted experiments that definitively established the law of conservation of mass. His experimental methodology was groundbreaking, shifting from qualitative observations to precise, quantitative analysis. You’ll see that Lavoisier meticulously measured the mass of substances before and after chemical reactions, ensuring that his scales were accurate to a fraction of a gram.
His experiments were simple yet effective. For example, when he heated mercury oxide, he found that the mass of the resulting mercury and oxygen gas precisely equaled the original mass of the mercury oxide. This was crucial. It demonstrated that mass was neither created nor destroyed; it merely changed forms within a closed system.
Here’s a simplified view of Lavoisier’s experimental approach:
| Experiment | Significance |
|---|---|
| Heating mercury oxide | Demonstrated mass conservation |
| Combining gases | Proved mass equivalence pre- and post-reaction |
| Calcination of metals | Illustrated mass changes and gas involvement |
| Decomposition of water | Confirmed additive mass properties |
| Combustion processes | Highlighted consistent mass relationships |
Each of these experiments reinforced the foundational principle of mass conservation, marking a pivotal shift from mystical alchemical practices to rigorous, empirical science. Through his work, you gain a deeper understanding of how meticulous experimentation leads to foundational scientific principles.
Key Principles Explained
You’ll find that the Law of Conservation of Mass asserts that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations.
The principle’s implications stretch across various scientific fields, influencing how you understand chemical equations and conservation in closed systems.
As you examine practical applications, it becomes evident how this law underpins procedures in chemistry and physics, ensuring mass consistency in both experimental and theoretical frameworks.
Mass Conservation Defined
Defining mass conservation, this principle asserts that the total mass in an isolated system remains constant regardless of the processes occurring within the system. You’ll find it essential to understand that this law underpins much of classical physics and chemistry, ensuring predictability in experiments and reactions.
Let’s delve deeper into how you can apply this concept with precision:
- Mass Measurement: Accurately measuring the mass of each component in a reaction or process is crucial. You need precise scales and techniques to ensure that mass is neither lost nor gained.
- Energy Equivalence: According to Einstein’s theory, mass and energy are interchangeable. This doesn’t contradict mass conservation but complements it. In chemical reactions, although energy forms may change, the equivalent mass must account for these energy changes.
- Isolated Systems: For mass conservation to hold, the system must be isolated—no mass can enter or leave. Understanding the boundaries of your system is key.
- Calculation Accuracy: Always double-check your calculations. Any error could lead to incorrect conclusions about the conservation in the system.
Practical Applications Explored
Exploring practical applications, let’s examine how the law of mass conservation operates in real-world scenarios to enhance your understanding and implementation of this fundamental principle. In fields like energy efficiency and waste management, the implications of mass conservation are both profound and pivotal.
Consider the design of modern industrial processes where energy efficiency is paramount. Here, understanding that mass is neither created nor destroyed but only transformed allows engineers to optimize systems for minimal waste production. This means that every bit of raw material is accounted for, whether converted into the product, by-products, or waste. Consequently, you’ll find that efficient planning and operation hinge on this principle to reduce costs and environmental impact.
In waste management, the conservation of mass principle informs the strategies for waste reduction, treatment, and recycling. By recognizing that all waste must go somewhere, professionals can innovate recycling techniques that convert waste back into usable materials, thus closing the loop. This not only conserves resources but also minimizes the ecological footprint of waste disposal.
Mass in Chemical Reactions
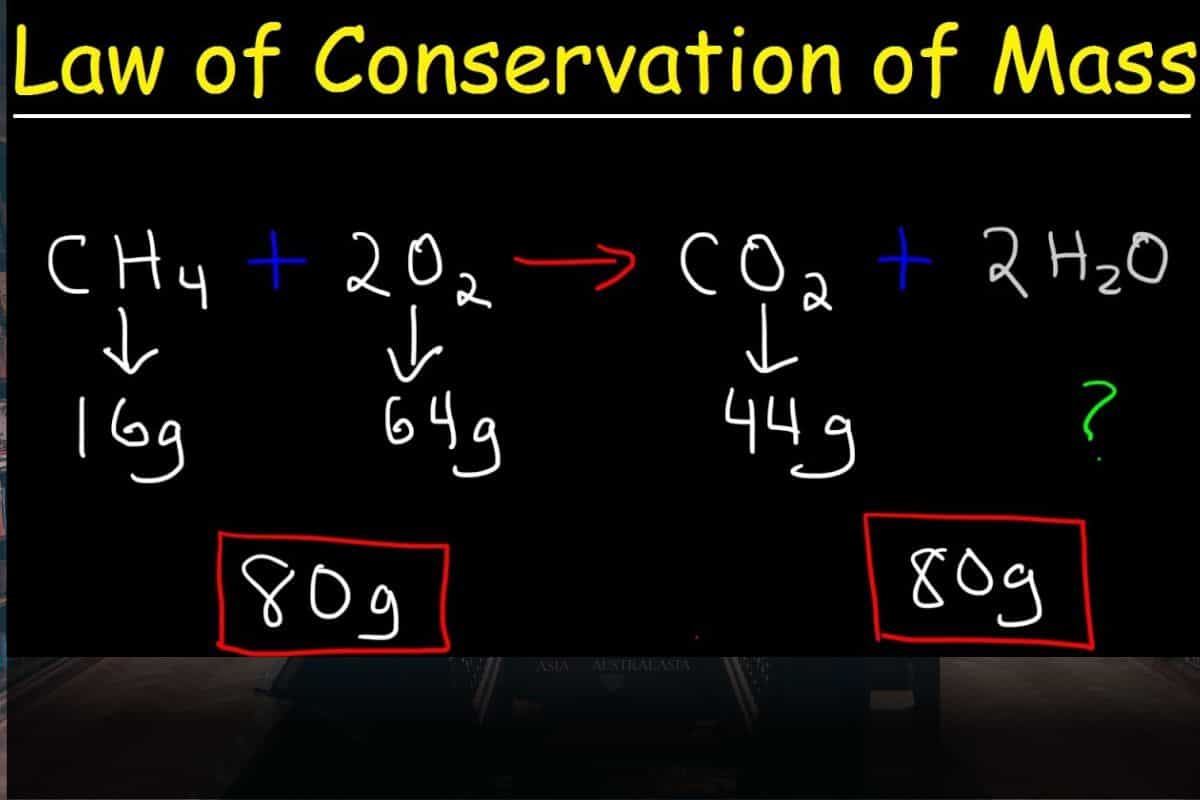
In chemical reactions, the total mass of the reactants equals the mass of the products, illustrating the law of conservation of mass. This fundamental principle asserts that mass can’t be created or destroyed within an isolated system. When you balance a chemical equation, you’re essentially accounting for all atoms involved in the reaction, ensuring that the mass on both sides remains equal.
Analyzing nuclear reactions introduces complexities such as isotopic variations which can affect the mass slightly due to differences in nuclear binding energies. However, even here, the law holds true when considering the system as a whole, including any energy changes as mass.
To understand the nuances, consider the following points:
- Atomic Accounting: Every atom in the reactants must be accounted for in the products.
- Energy Considerations: In nuclear reactions, energy released or absorbed alters the apparent mass, reflecting Einstein’s equation \(E=mc^2\).
- Isotopic Variations: Isotopes of the same element may react differently due to variations in their nuclear structures.
- Closed System Requirement: The law applies strictly in closed systems where no mass enters or leaves the reaction environment.
Common Misconceptions
You might often hear that mass can appear or disappear, but this is a fundamental misunderstanding of the Law of Conservation of Mass.
In reality, the total mass of a closed system remains constant, regardless of the processes occurring within.
The misconception that mass can be created leads to significant confusion when analyzing chemical reactions and energy transformations.
Mass Creation Myth
Many people mistakenly believe that matter can be created from nothing, contradicting the law of conservation of mass. This misconception often stems from mythological origins and cultural interpretations that metaphorically suggest creation ex nihilo (from nothing).
You might find ancient texts filled with stories where gods conjure the cosmos and all within it instantaneously. However, in the realm of physics, these narratives don’t hold water.
Here’s why the idea that matter can spontaneously appear is a fallacy:
- Mass Conservation: The total mass in an isolated system remains constant over time. No physical process can increase or decrease it.
- Energy Equivalence: According to Einstein’s theory of relativity, mass and energy are interchangeable, yet neither can be outright created or destroyed.
- Closed Systems: In closed systems, such as the universe, the total amount of matter and energy remains constant.
- Chemical Reactions: In reactions, atoms rearrange, but the mass of reactants equals the mass of products, illustrating mass conservation.
Understanding these principles helps clarify that what might seem as ‘creation’ of mass is merely transformation or transfer. It’s crucial to distinguish literal interpretations from metaphorical or philosophical ones to avoid conflating scientific facts with fiction.
Total Mass Misunderstanding
Common misconceptions often cloud people’s understanding of the law of conservation of mass, suggesting inaccurately that mass can appear or disappear. It’s crucial to recognize that mass measurement errors or changes in physical states might lead you to believe mass is lost or gained. However, this isn’t the case. The law asserts that in a closed system, the total mass remains constant, regardless of the transformations that occur within.
To gain conceptual clarity, consider a burning piece of paper. You might think mass is lost when the paper turns to ash and smoke. However, if you were to capture all the smoke, ash, gases, and even the heat energy released, you’d find the total mass unchanged. The apparent loss is merely a redistribution of mass into forms that aren’t always easy to measure without precise instruments.
Understanding this requires precision in both thought and practice. Mass measurement isn’t merely about placing objects on a scale; it involves accounting for all forms of output in a reaction. This precision ensures that you don’t fall into the trap of assuming mass can vanish or emerge from nowhere.
Embrace rigorous methodologies in your observations and calculations to uphold the integrity of this fundamental law.
Conservation in Closed Systems
In closed systems, mass remains constant as reactions and processes occur within their boundaries. This principle hinges on the fact that no matter is lost or gained; it merely transforms. You’ll see that energy transfer plays a crucial role within these confines, as it governs how substances shift phases or react chemically without altering the total mass.
Understanding the interaction between energy transfer and system boundaries illuminates why mass conservation is integral to closed systems:
- Energy Transfer: It facilitates reactions by converting energy forms, yet doesn’t contribute to mass change.
- System Boundaries: These are impermeable to matter, ensuring that all mass involved in the reactions stays within the system.
- Phase Changes: Even when a substance changes from solid to liquid or gas, the total mass remains unchanged.
- Chemical Reactions: Atoms rearrange in new configurations; however, the sum of the masses of reactants equals the mass of the products.
Grasping these points, you recognize that in closed systems, the law of conservation of mass dictates that total system mass is invariant regardless of the internal changes. This principle isn’t only fundamental in theoretical chemistry but also essential for practical applications in understanding and designing chemical processes.
Real-World Applications
Let’s explore how the law of conservation of mass applies to various industrial and environmental processes. In industries, understanding this law is crucial for optimizing production methods and developing more sustainable practices.
For instance, mass recycling initiatives hinge on this principle. In a recycling process, waste materials are transformed into new products. The mass of the recycled products must equal the mass of the original materials, minus any losses due to processing. This ensures that materials are used efficiently, minimizing waste and conserving resources.
Energy efficiency in manufacturing is another area where the law of conservation of mass is pivotal. By analyzing the mass flow in chemical reactions, industries can optimize processes to maximize yield and minimize energy consumption. The accurate calculation of reactants and products in a reaction ensures that all mass is accounted for, reducing unnecessary energy expenditure in the form of heat or unreacted materials.
This not only supports cost reduction but also enhances environmental sustainability by decreasing resource depletion and emissions.
Thus, the integration of the law of conservation of mass in these areas isn’t just a theoretical exercise—it’s a practical necessity for advancing energy efficiency and mass recycling in a world striving for sustainability.
Also Read
- Malibu Magic: Cruise the Scenic Pacific Coast Highway
- What Is This Case of Isaac Soleimani and INE Soleimani LP V. Andre Hakkak, Et Al
Impact on Environmental Science
You’ll find the law of conservation of mass fundamental in shaping modern environmental science, particularly in studies of ecosystem dynamics and pollution control. By understanding that mass in an isolated system is neither created nor destroyed, you can better comprehend how pollutants distribute within ecosystems and the implications for sustainability and management.
Here’s how this law is pivotal in various environmental science contexts:
- Ecosystem balance: It helps in quantifying the changes in the concentration of nutrients and pollutants, ensuring that total mass remains constant throughout the process. This aspect is crucial for predicting the impacts of human activity on natural environments.
- Pollution tracking: By applying this law, scientists can trace the pathways of pollutants in the environment, understanding their origin, transformation, and ultimate fate.
- Waste management: This principle guides the development of strategies for waste reduction and conversion, enhancing recycling efficiency.
- Climate studies: It’s instrumental in calculating the budgets of carbon and other greenhouse gases, which are critical for assessing global warming potential and designing mitigation strategies.
Experimental Demonstrations
To fully grasp the law of conservation of mass, consider its practical implications through various experimental demonstrations. Engaging in classroom activities or DIY experiments provides invaluable firsthand experience of this foundational principle.
For instance, you might conduct a simple experiment involving the chemical reaction between vinegar and baking soda. Here, you’ll measure the mass of both reactants separately and then together in a closed system to observe that, despite the reaction and the generation of gas, the total mass remains unchanged.
Another effective demonstration involves heating a sealed flask containing a small amount of water. As the water evaporates and condenses back into liquid, you can measure the flask at different stages of the process. You’ll find the mass remains constant, illustrating that mass is conserved in both physical and chemical changes.
These experiments underscore the precision required in scientific measurements. Accurate scales are crucial, as even slight inaccuracies can lead to misunderstandings about mass changes.
It’s essential to ensure all reactants and products are contained within the system being studied, as any loss to the surroundings, like gas escaping from an unsealed container, would compromise the integrity of the experiment.
Further Reading Suggestions
For a deeper understanding of the law of conservation of mass, consider exploring these recommended texts and articles. Delving into these educational resources will enhance your grasp of both fundamental principles and advanced topics in chemical and physical processes.
You’ll find that the following books and articles offer a blend of theoretical explanations and practical illustrations:
- ‘Chemical Principles: The Quest for Insight’ by Peter Atkins and Loretta Jones – This textbook dives into chemical reactions with a focus on atomic structure and the conservation laws that govern chemical processes.
- ‘Modern Chemistry’ by Raymond Chang – Chang’s comprehensive approach helps clarify complex concepts including the law of conservation of mass through rigorous examples and problem-solving exercises.
- ‘Fundamentals of Physics’ by David Halliday, Robert Resnick, and Jearl Walker – While primarily focused on physics, this book provides invaluable insights into the conservation laws that overlap with chemistry, particularly in thermodynamic contexts.
- ‘The Journal of Chemical Education’ – Regularly features articles and papers that explore innovative teaching methods and experimental demonstrations related to the conservation of mass.
Frequently Asked Questions
How Does Conservation of Mass Relate to Energy Conservation?
In thermodynamic systems, you’ll find that mass conservation ties closely to energy conservation. During chemical reactions, mass isn’t lost but converted, aligning with energy’s behavior as dictated by the first law of thermodynamics.
Can Mass Change Into Energy Under Any Conditions?
Yes, mass can change into energy under certain conditions, such as in nuclear reactions due to mass-energy equivalence, where mass and energy are interchangeable, precisely described by Einstein’s equation, E=mc^2.
What Are the Limitations of Conservation of Mass in Physics?
In physics, the conservation of mass has limitations during nuclear reactions, where mass can convert into energy, unlike in chemical reactions, where mass remains constant. You’ll find mass isn’t strictly conserved in these scenarios.
How Does Quantum Mechanics Challenge the Conservation of Mass?
In quantum mechanics, you’ll find that particle behavior defies classic conservation laws; mass can convert into energy, illustrating energy equivalence, which challenges the strict application of mass conservation in subatomic interactions.
Is the Law of Conservation of Mass Applicable in Black Holes?
In black holes, the law of conservation of mass faces challenges due to event horizon mysteries and gravitational singularity effects, where traditional physics breaks down and mass-energy conversion isn’t clearly understood.
Conclusion
You’ve explored how the law of conservation of mass underpins numerous scientific principles and practical applications. This fundamental concept ensures that mass is neither created nor destroyed in an isolated system. It is pivotal in fields from chemistry to environmental science.
By understanding and applying this law, you can predict the outcomes of chemical reactions and address environmental challenges more effectively.
Continue delving into this topic through advanced studies and experiments to further enhance your grasp of its extensive implications.